Jun 10th 2025
Acetic Acid a Colorless Liquid Organic Compound
Acetic Acid: The Colorless, Odorless Liquid Organic Compound from the Family of Carboxylic Acids Pioneering Innovation
Acetic acid, a carboxylic acid, is a leader in the area of organic chemistry. It is the sole component in your kitchen vinegar that has a strong, distinctive smell, it is also the main ingredient in industrial synthesis and laboratory research, all of which utilize this colorless liquid organic compound. It is a silent worker that drives a number of processes and products we consume every day without even realizing it. For Engscientific, getting the best quality acetic acid and understanding it is critical to the powers of innovation across the different sectors.
What is Acetic Acid? The Core Concept
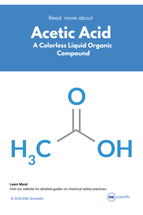
The acetic acid is the main chemical agent in the vinegar and it is known as ethanoic acid in the chemical world. Its formula is CH3 COOH. It is the simplest carboxylic acid after formic acid that is known for its sour taste and strong smell. It is colorless and has no odor at room temperature. It is also completely soluble in water, ethanol, and diethyl ether. These properties are derived from the presence of a carboxyl group (−COOH) in its molecular structure. This group imparts weak acidic characters to the compound, which allows it to act as a proton donor in redox reactions.
It is "acetic" in the name that comes from the Latin word acetum, which means vinegar, a fact that vividly illustrates its most famous natural form where it usually constitutes a 3–9% solution that is obtained by the fermentation of ethanol by Acetobacter bacteria. The first known use of this substance hence traced back to this ancient discovery, but its role as the key chemical substance today has been growing rapidly since then.
Natural Occurrence and Industrial Production
Apart from the fact that it's the major component of vinegar, acetic acid carbon is also sourced from nature in places such as some fruits, vegetables, that are metabolically active or even living beings which are turned into metabolic wastes. The compound is at the center of both aerobic and anaerobic metabolism processes of microorganisms.
That said, however, due to the very nature of the case, the acetic acid needs to be produced in large quantity which can only be achieved through the industrial route. The following methods are instrumental:
- Methanol Carbonylation (Monsanto and Cativa Processes): This is the predominant industrial route. Methanol (CH3 OH) reacts with carbon monoxide (CO) in the presence of a catalyst (rhodium for Monsanto, iridium for Cativa) to directly synthesize acetic acid. These processes are highly efficient and produce a very pure product.
- Oxidation of Acetaldehyde: Acetaldehyde (CH3 CHO) can be oxidized with oxygen in the presence of metallic catalysts (e.g., manganese or cobalt acetates) to yield acetic acid. While historically significant, this method is less common for large-scale primary production compared to methanol carbonylation.
- Fermentation: While traditionally used for vinegar, industrial-scale fermentation processes can also produce acetic acid. Anaerobic bacteria like Clostridium thermoaceticum can convert various feedstocks (e.g., glucose, syngas) into acetic acid. This method is gaining renewed interest for its potential use of renewable resources.
Key Chemical and Physical Properties
The performance of acetic acid depends on its strong group of properties:
- Weak Acid: It is very corrosive in concentrated form but acetic acid is a weak acid, meaning it only partially ionizes in water. Its pKa is about 4.76.
- Polarity: The carboxyl group is polar while the methyl group is nonpolar in the molecule of acetic acid and, therefore, it can dissolve both polar and some nonpolar substances.
- Boiling Point: At 118∘C (244∘F), it is the temperature of its vaporization which is quite high considering the molecular weight of the substance due to the strong hydrogen bonding among the molecules.
- Freezing Point: Pure acetic acid free of water (glacial acetic acid) freezes at 16.6∘C (61.9∘F), which is slightly lower than usual room temperature. It also means if the temperature goes down a bit it is going to "freeze" or crystallize like ice.
- Reactivity: As a carboxylic acid, it goes easily through esterification (reaction with alcohols to give esters) it also constitutes forming salts (acetates) and it may be reduced to alcohols or aldehydes besides in addition to various organic synthesis reactions.
Diverse Applications Across Industries
Acetic acid is very versatile and it fits perfectly as the key player in a wide variety of industries:
- Food Industry: This is the case with vinegar where it is the flavor that shall never be missing and also it is the natural preservative. Moreover, it is the acidulant in condiments, pickles, and baked goods. Sodium diacetate, a complex of acetic acid and sodium acetate, is a common bread preservative.
- Chemical manufacturing: This is definitely the biggest user of acetic acid. It is the main raw material for:
- Vinyl Acetate Monomer (VAM): The building block for polyvinyl acetate (PVA), used in paints, adhesives, and textile finishes.
- Acetic Anhydride: A key reagent for acetylating cellulose to produce cellulose acetate, used in photographic films, textile fibers, and cigarette filters.
- Esters: Numerous acetate esters (e.g., ethyl acetate, butyl acetate) are widely used as solvents in paints, coatings, inks, and as flavorings/fragrances.
- Purified Terephthalic Acid (PTA): A crucial intermediate in the production of PET plastics used in bottles and polyester fibers.
- Pharmaceuticals and Cosmetics: Used as a solvent for various reactions, in the synthesis of drugs like aspirin (acetylsalicylic acid), and as an active ingredient in some topical medications. It's also found in some cosmetic formulations.
- Textile Industry: Employed as a dyeing aid and in the production of synthetic fibers like acetate rayon.
- Cleaning Agents: Due to its acidic nature, it is a great descaling agent, rust remover, and general household cleaner.
- Laboratory Reagent: A major ingredient in various analytical tests, buffer solutions, and organic synthesis reactions in research and educational institutions.
Safety and Handling Considerations
Though highly beneficial, concentrated acetic acid is a corrosive substance, which can lead to severe injuries if it comes in direct contact with the skin or eyes. The vapors can also cause an irritation in the respiratory tract. The use of proper PPE– gloves, eye protection, and laboratory coats – and sufficient ventilation are indispensable when handling it.
Engscientific: Your Partner for Quality Acetic Acid
Engscientific is committed to providing you with high-purity acetic acid of the highest quality that meets your needs in R&D, production, or quality control. We have a mission to deliver this essential chemical with the highest standards, which allows you to use it with confidence, and ensures that quality and performance are maintained. The various quality control procedures we perform as well as being responsible in the distribution and supply chain make Engscientific a reliable partner for all your chemical requirements.
Acetic acid is no longer only the main ingredient of vinegar but has become a major player in almost every industry. The colorless liquid organic compound will be there as ever for innovation and processes leading the way.